Besides the intrinsic importance of the discovery, I would like to briefly recall - I have developed elsewhere - an equally important point.
The NASA Viking Mars landers, in 1976 and 1977, were the only probes so far to land on Mars with equipment aimed at detecting traces of life.
Viking's LR experiment consisted of sampling Martian soil, adding nutrients to it and checking if gas, a sign of metabolism, was being produced.
This was strongly the case, and it was originally reported that there is life on Mars.
Then one rejected this result with three main arguments. One was that it must have been some chemical activity, not metabolic activity. One just forgot that after sterilization in the LR experiment, the signs of metabolism had disappeared from the sample; the sterilization procedure which was precisely planned to avoid confusing chemistry and metabolism.
The other was that another experience aboard Viking had unconclusive results, and another had been negative. One had forgotten that the reason there were several different experiences, was not because it was decided that they all had to give positive results, but because it was estimated that since a Martian life could not espace detection because of its unknown nature, it was better to multiply the chances of detection by performing different experiences.
The third argument was that the experience that tried to detect organic material on Mars did not find any. This time, it goes without saying that it was quite reasonable to say that if there was no organic matter, there was no life. But...
On the one hand, tested again on Earth, the experience that detected no organics on Mars did not find any on Earth either. The experience had simply been a technical failure, not proof of the absence of organic matter on Mars.
On the other hand, and this article is a proof of that, there is indeed organic matter on Mars.
Therefore, in view of this and a few other facts uncovered since then, Viking's allegedly negative result should have been reconsidered. Few scientists have been willing to admit it.
By Caroline Freissinet, post-doctoral researcher at the NASA Goddard Space Flight Center, SAM team.
The Sample Analysis at Mars (SAM) instrument team confirmed the in situ detection of chlorinated organic molecules in a Martian rock sample. SAM (Figure 1) is one of ten active instruments of the Curiosity rover of the Mars Science Laboratory (MSL) mission. Curiosity now surveys the base of Mount Sharp in Gale crater where it landed in August 2012. SAM, for which Paul Mahaffy from the NASA Goddard Space Flight Center in Maryland is in charge, is a real chemical laboratory that also analyzes both the composition of the atmosphere and that of soil and rock samples, providing information on their mineralogy and organic matter content. It was on a sample obtained from drilling a rock in the geological formation of Yellowknife Bay (Figure 2) that SAM revealed the presence of organic molecules. While these chemical species, made up of at least carbon and hydrogen atoms, are the building blocks of all life on earth, organic molecules can also be produced by abiotic chemical reactions. However, the team's results do not make it possible to differentiate between a biological or geochemical origin of the organic matter discovered.
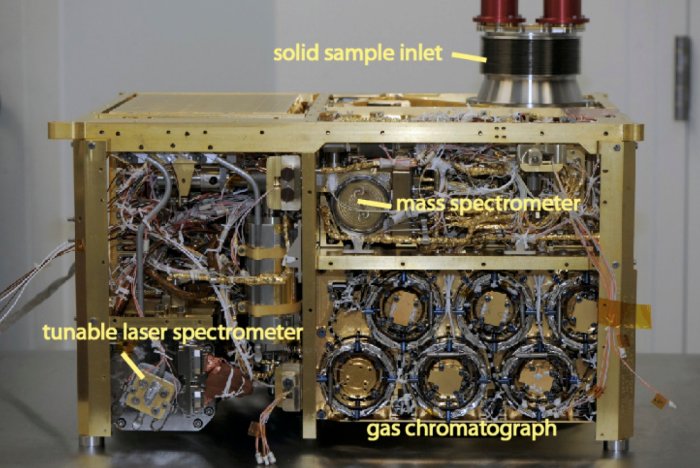
Figure 1: The SAM instrument which detected Martian organic molecules is subdivided into three instruments: the adjustable laser spectrometer for the measurement of isotopes and methane, the gas chromatograph for the separation of molecules and the mass spectrometer for their identification. Image credit NASA-GSFC.
Whereas the surface of Mars appears now to be an inhospitable environment for life, it is now believed that the Martian atmosphere was denser and that liquid water was perennial on its surface in the distant past. The latest results of the MSL mission notably demonstrated the presence of a vast lake 150 km in diameter filling the Gale crater. Mars then seems to have been warmer and wetter in the past, and the presence of organic molecules only completes the list of ingredients necessary for the emergence of a life form. These reduced molecules are both building blocks and energy sources that a simple life form can use to develop. It is believed that on Earth life appeared around 3.8 billion years ago, and our results show that at least some areas of Mars had similar conditions to those on Earth at that time. If life could have developed under these conditions on Earth, then why not also on Mars?

Figure 2: Self-portrait of Curiosity in Yellowknife Bay. The hole resulting from the first drilling (John Klein) is visible on the rock, next to the test hole. Cumberland was subsequently drilled 2.75 m from John Klein. Image credit NASA / JPL-Caltech / MSSS
The organic molecules detected by the team are found in a sample at the site called Cumberland (Figure 3). The sample was taken by drilling in a clay rock 4 billion years old and composed of 20% smectite. Clays are considered to have good properties of concentration and preservation of organic matter, due to their large contact surface and their available spaces between the layers. However, the presence of oxidants in soils, ultraviolet radiation at the surface, and the penetration of cosmic and solar radiation underground (up to 2-3 meters deep) make the surface of Mars a hostile environment for organic molecules. But despite being 4 billion years old, the Cumberland sample has only been exposed to cosmic and solar radiation during its excavation in the past 78 million years (Farley et al. 2014), fortunately for the preservation of organic molecules!

Figure 3: Map showing Curiosity's journey from its Bradbury landing site to Pahrump Hills at the base of Mount Sharp. The shoveled (Rocknest) and drilled (John Klein, Cumberland and Confidence Hills) sampling sites are shown. Detection of organic molecules endogenous to the sample was positive in Cumberland only. Image credit Fred Calef, NASA / JPL-Caltech, Caroline Freissinet.
The organic molecules detected at Cumberland are all chlorinated: chlorobenzene, dichloroethane, dichloropropane and dichlorobutane (Figure 4). Chlorobenzene is most abundant with concentrations ranging from 150 to 300 ppbm (parts per billion, by mass). Although the presence of these molecules in this form is possible in the Martian sample, it is rather considered that organic precursors were present in the sample, and that they were chlorinated by reaction with perchlorates during the phase of pyrolysis (heating) of the sample in the SAM oven. Perchlorates (a chlorine atom bonded to 4 oxygen atoms) are extremely powerful oxidants when heated, and are believed to be found all over the planet today. In addition to O2 they release which will consume the molecules, perchlorates can also release hydrogen chloride (HCl) and chlorine (Cl2). These chlorinated molecules are highly reactive under the effect of heat, and react with the organic molecules present in the sample to form the chlorinated organic species detected by SAM. The identity and origin of the precursor molecules remain uncertain; they could have been formed on Mars by hydrothermal, atmospheric or biological processes, or else brought by meteorites, micrometeorites or comets. The simultaneous presence of perchlorates and organic molecules increases the interest of perchlorate reducing bacteria, which derive their energy from the redox cycle of chlorine, such as Dechlorimonas agitates or Paracoccus halodenitrificans, and which could be proof of the capacity of living organisms to live in the chemical conditions prevailing on the surface of Mars.
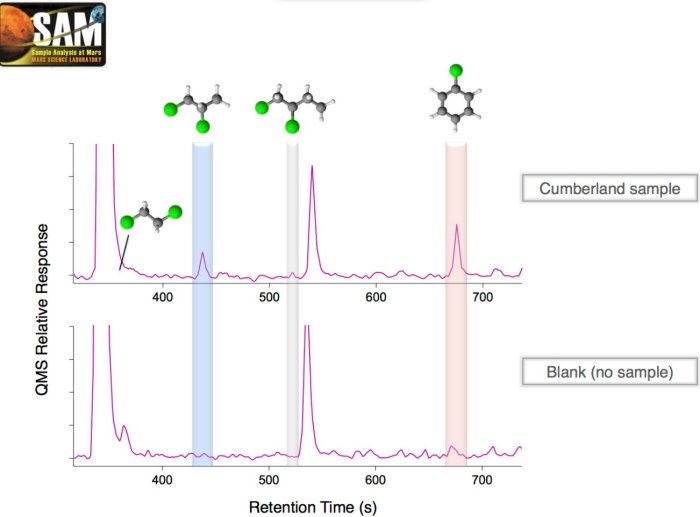
Figure 4: Comparison of a chromatogram obtained for a Cumberland sample with the chromatogram resulting from a blank (same experiment without sample). Chlorinated organic molecules composed of 2 to 6 carbon atoms are present in the sample at significantly higher abundances than in the blank, attesting to the endogenous nature of these molecules to the Martian sample. Image credit Caroline Freissinet.
The detection and identification of organic molecules were carried out by mass spectrometric analysis of the gases released by the sample as a function of its temperature, and by gas chromatography coupled with mass spectrometry (Figure 4), two SAM detection modes. The soil samples analyzed consisted of ~ 45 mg of rock crushed and sieved to particle sizes < 150 mm, and were heated to 900 °C. The first positive signal for chlorobenzene and dichloroalkanes was obtained on the ground (Martian day) 290, on May 30, 2013, during the analysis of the 3rd sample from Cumberland, after certain analytical parameters of the SAM instrument were changed. This detection was confirmed in subsequent analyzes (Figure 5). It is important to note here that chlorobenzene had already been detected for all analyzes of solid samples, but at a low and constant background level of 3 to 10 pmol, well below the abundances detected in the Cumberland samples. This is one of the reasons why the SAM team spent more than a year carefully analyzing the results, carrying out laboratory studies aimed, among other things, at understanding in detail the possible carbon sources intrinsic to the instrument (therefore terrestrial), before being able to conclude with certainty that the detection came from the sample, therefore from Mars. As Danny Glavin, SAM team scientist at NASA-GSFC and second author of the forthcoming article in Journal of Geophysical Research: Planets, says: "Finding organic molecules on Mars is extremely difficult. The first step is to identify the environments of the Gale crater likely to concentrate organic molecules in the sediments. These molecules must then survive the transformation of these sediments into rocks (diagenesis), where the percolation of fluids and various dissolved substances can oxidize and destroy organic molecules. These molecules can then be destroyed when rocks are exposed to intense ionizing radiation and oxidants from the surface and sub-surface of Mars. Finally, to be able to identify the organic compounds that will have survived until then, we must deal with the presence of perchlorates and other potential oxidants in the sample, which will react and / or consume the organic molecules in carbon dioxide and form chlorinated hydrocarbons when samples are heated in SAM."
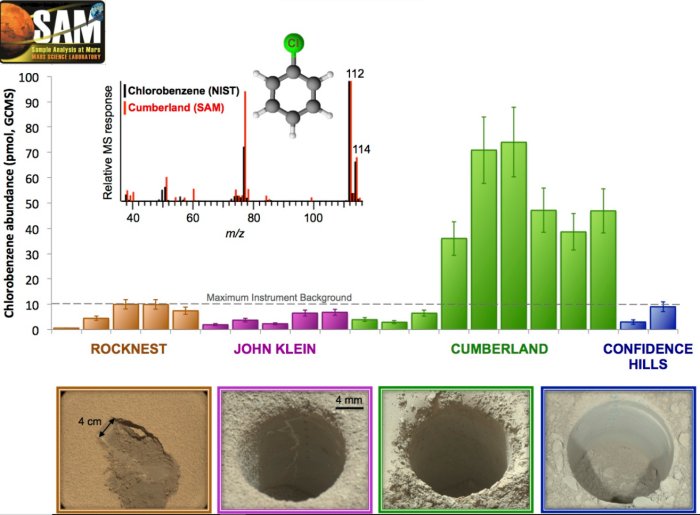
Figure 5: Quantity of chlorobenzene (in picomoles) detected during the various analyzes of soils and rocks taken by Curiosity: chronologically Rocknest, John Klein, Cumberland and Confidence Hills. The presence of chlorobenzene above the background level is attested at Cumberland. This quantity falls back to the background level in subsequent analyzes, validating a detection of organic molecules in the sample at Cumberland. The mass spectrum of chlorobenzene (black, NIST) is compared to that obtained with SAM (red) as an insert. Image credit Caroline Freissinet / NASA / JPL-Caltech / MSSS.
The discovery of these organic molecules in an ancient rock opens up new perspectives in the search for more complex molecules. Besides the importance it has in providing the context of habitable environments and finding a localized area favorable to the emergence of a life form, it also allows us to better understand where to look in the future, which sedimentary rocks representing particular environments are the most favorable for the accumulation and preservation of organic material. If chemical traces of life were once produced on Mars, we now know that they are likely to persist there until today, and can be found and studied there by present and future Martian exploration robots. The challenge now for Curiosity is to find other target rocks on Mount Sharp, which would have a different inventory and richer in organic compounds.
This projection into ancient Mars is an important step in understanding the presence and diversity of prebiotic or biotic molecular signatures in extraterrestrial environments. The recent announcement of localized release of methane in the Martian atmosphere (Webster et al., Science 2014), as well as the detection of organic molecules in a rock, suggest that the red planet has been and is much more active than did not think so until then. But how far will Mars continue to surprise us?
Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars.
C. Freissinet, DP Glavin, PR Mahaffy, KE Miller, JL Eigenbrode, RE Summons, AE Brunner, A. Buch, C. Szopa, PD Archer Jr, HB Franz, SK Atreya, WB Brinckerhoff, M. Cabane, P. Coll, PG Conrad, DJ Des Marais, JP Dworkin, AG Fairén, P. François, JP Grotzinger, S. Kashyap, IL ten Kate, LA Leshin, CA Malespin, MG Martin, FJ Martin-Torres, AC McAdam, DW Ming, R. Navarro -González, AA Pavlov, BD Prats, SW Squyres, A. Steele, JC Stern, DY Sumner, B. Sutter, MP. Zorzano, and the MSL Science Team.
Note:
By reading the article, one may somehow logically wonder where the perchlorates were the chemical, rather than metabolic, cause of the postive Viking LR experiment. Not so:
However, perchlorates cannot account for the Viking LR data because perchlorates are stable under all thermal conditions of the Viking LR experiment, including the 160°C control (Quinn et al., 2013).
Source: