| Life on Mars Main | Order Papers | Mars Pictures | Viking Mission | Comments and Questions |
| Life on Mars Main | Order Papers | Mars Pictures | Viking Mission | Comments and Questions |
|
ABSTRACT |
*<gillevin@biospherics.com>, <http://www.biospherics.com>
Keywords: Labeled Release Experiment, Viking Mission to Mars, Life on Mars, Mars, Microorganisms on Mars, Extraterrestrial Life
|
Fresh speculation about life on Mars was aroused by reports of possible biochemical and microbiological fossil evidence of life found in meteorites ALH840011 and EETA790012, generally accepted as of martian origin. Together with other developments concerning life on Mars, a re-examination of this major scientific issue is warranted. In 1976, the Viking Mission Labeled Release life detection experiment (LR) returned data from Mars fully satisfying the pre-mission criteria for the detection of microbial metabolism. Nonetheless, the consensus of scientists interpreting the Viking results was that the surprising activity the LR detected in the martian surface material should be attributed to chemistry and not biology. 1.1 Principal challenges to biological interpretation of LR data 1. No organic compounds were found in martian soil analyzed by the Viking Gas Chromatograph Mass Spectrometer (GCMS). 2. H2O2 , chemically formed in the upper atmosphere, was thought to descend to the soil and, directly or through forming complexes or compounds, to oxidize the LR substrates to evolve labeled gas. 3. It was assumed that there is no liquid water on Mars, and that its absence would make life impossible; and, as a corollary, the overall environment was believed too extreme to support life. 4. The amplitude and kinetics of the LR response from Mars was thought to be "too much too soon" for any putative martian biology. 5. Although not a pre-mission criterion for life, a second injection of LR nutrient onto positive samples failed to re-invigorate the evolution of gas, as generally occurs with terrestrial soils. Instead, some of the gas evolved after the first injection appeared to be reabsorbed into the soil. 6. No visual evidence for life was reported when the Viking camera images were examined. 7. UV light from the sun was thought to activate soil particles, which then disrupted the LR nutrient upon contact, releasing labeled gas. Also, UV light's destructive effect was held to account for the reported lack of any organic matter on Mars. 8. Clays on Mars were proposed to react with the LR nutrient to release labeled gas. 9. A non-biological explanation of the Mars LR response seemed far simpler than proposing a separate origin of life on Mars. Application of Ockham's Razor, therefore, indicated chemistry or physics over biology. For one or more of the reasons stated above, the
majority of involved scientists believed that the LR response was non-biological.
However, the bases for that belief have been weakened. Over the intervening
years since Viking, many new facts have emerged. Re-examination of the
data and relevant scientific discoveries, including the evidence for life
in the martian meteorites, requires a new evaluation. Each of the reasons
enumerated above to support the non-biological rationale will be examined
under new light.
NASA selected three life detection experiments for Viking based on three different assumptions about possible martian life. Therefore, it was explained, were there life on Mars, it would be likely that only one of the experiments would detect it. 2.1 The LR life detection experiment A brief review of the Viking LR experiment is needed for the background to this discussion. The LR applied a small drop of nutrient solution to the center of a soil sample. The liquid spread outward, providing a wet-to-moist gradient through the sample. Organic substrates in the nutrient were at very low concentrations to prevent possible toxicity. For the same reason, no co-factors, growth supplements, or inorganic compounds were added (it was presumed that the soil already would have the ingredients required to sustain any life present). No buffer was used so that the sample itself would determine the pH. A slight helium overpressure and a temperature of 10°C were maintained to assure liquidity of the water during the experiment. Phase separation by gravity provided a signal free from the noise of the radioactivity still retained in the liquid. The LR experiment was the simplest and the most sensitive to microorganisms by orders of magnitude. Unlike the Pyrolytic Release (PR) experiment, it required no intervention (an optical filter) to prevent a false positive; unlike the Gas Exchange (GEx) experiment, it did not adjust the raw data (factoring in presumed coefficients of the martian soil and adjustments for probable gas sample size) prior to interpretation. The LR experiment3,4 is based on the widespread metabolism by microorganisms of Miller-Urey compounds5, which are presumed to have been available for pre-biological evolution on primitive Earth. Selection of a particular compound was made after its metabolism was confirmed in each of a wide variety of microbial species in pure and mixed cultures and in soils. Designated VM1, the LR nutrient solution was an aqueous solution of 2.5 x 10-4 molar concentrations each of formate, glycine, D-alanine, L-alanine, D-lactate, L-lactate, and glycolate, each uniformly labeled with 14C. The LR experiment moistens a 0.5 cc soil sample with 0.115 ml of VM1 for a total of 3.91 uCi per application. Evolution of 14C gas from the moistened soil indicates microbial activity. Following such a positive response, a duplicate of the original sample is heated to impair or destroy any organisms present. After cooling, that sample is tested exactly as was the one that produced the positive result. A positive response from the second sample indicates that the response of the first was non-biological in nature, while a negative response, demonstrating that the causitive agent had been deactivated by heat, completes the evidence for life. The temperature regimen selected by the Viking Biology Team to distinguish a biological response from a non-biological one was 160o C for 3 hr. Thousands of soils and microbial cultures of a wide
variety of species were tested. No sample with demonstrated living organisms
failed to respond. The controls readily confirmed biological activity.
(Only several of the hundreds of soils were sterile.) The metabolism of
as few as 50 cells in lag phase was readily detected. In a final test of
an LR flight instrument, 0.5 ml of a California soil was placed under the
experimental conditions to prevail on Mars. After 3 days of the reduced
atmospheric pressure, humidity and temperature, the LR substrate solution
was injected. Radioactive gas immediately evolved and increased through
the standard 8-day LR test period (see SN103, FIG. 6). A second injection
of nutrient then produced an increased response. A duplicate of the test
soil was heated to 160o C for 3 hours and, after cooling, was tested. Essentially
no
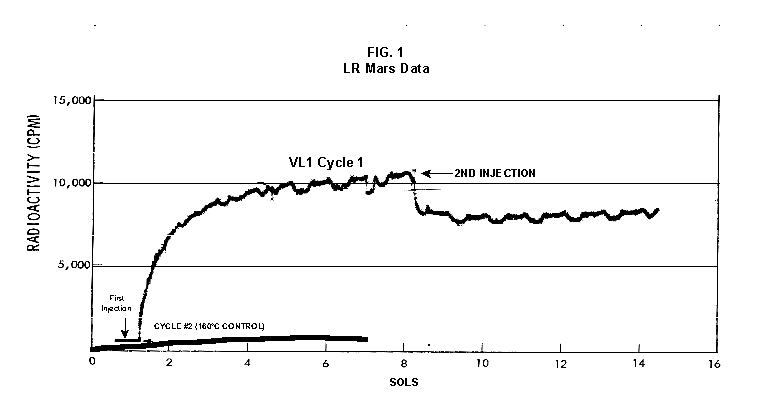
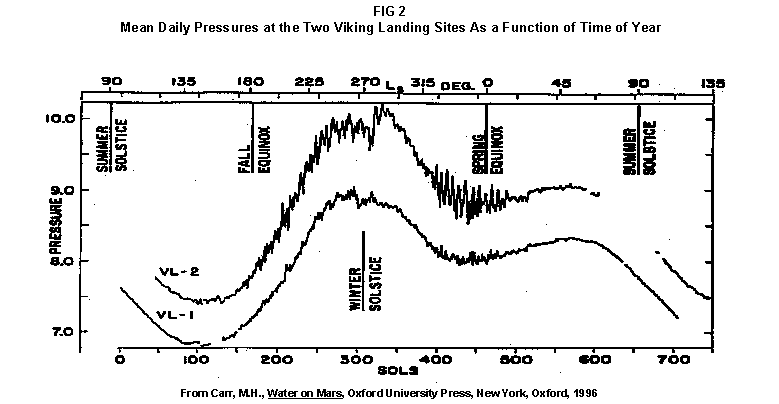
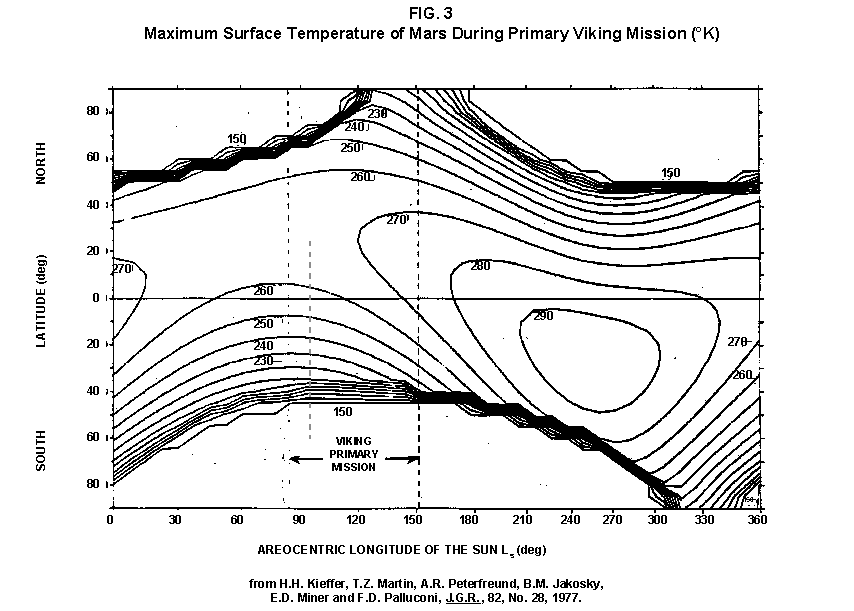
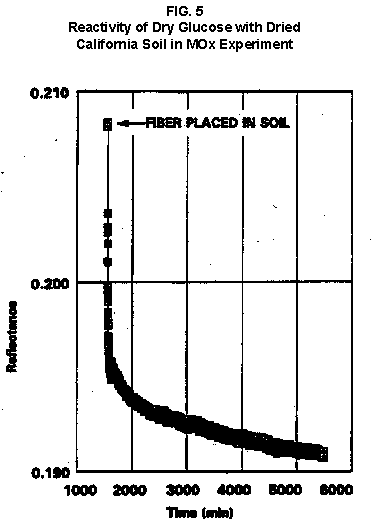
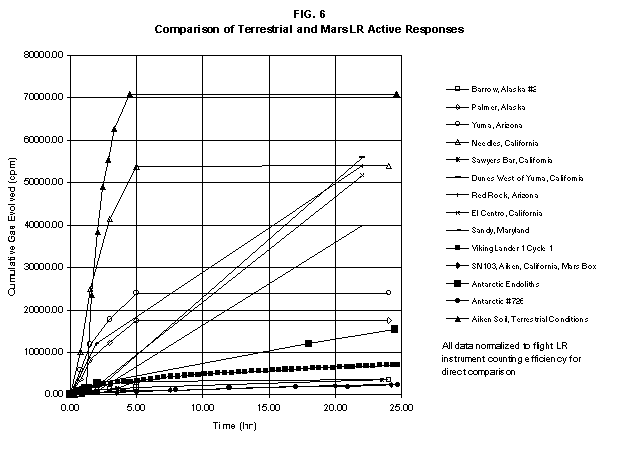
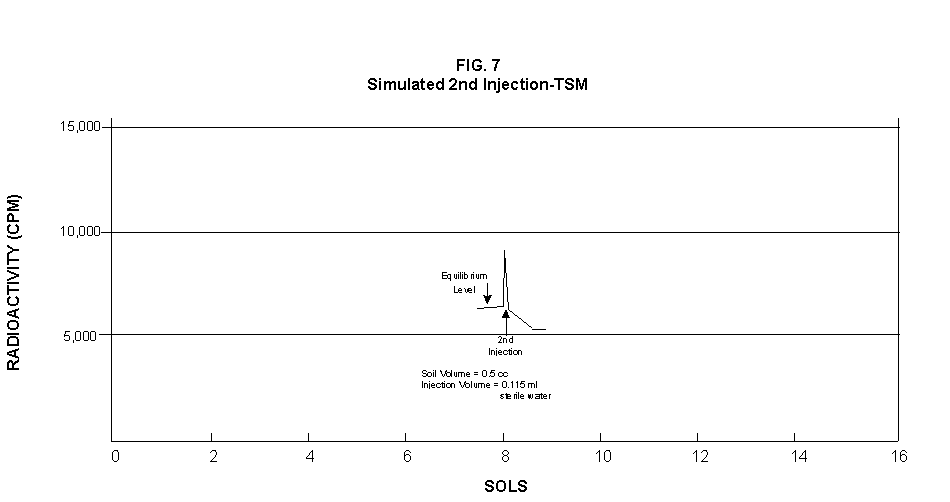
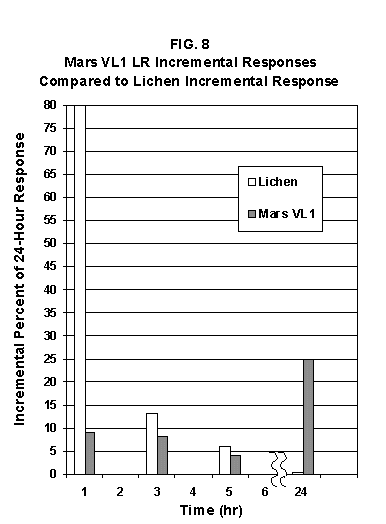
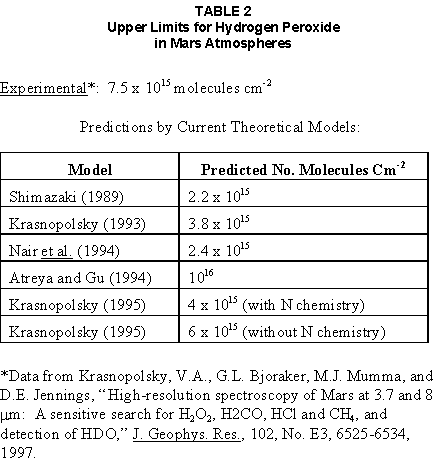
2.2 The LR on Mars The results from the first LR experiment at the Viking 1 Site on Mars are shown in FIG. 1. At the Viking 2 Site, some 4,000 miles away, the soil produced a similar positive response. After eight sols (one martian sol = 24.67 Earth hr) of continuing, but slowing, evolution of gas, the soil samples at both sites were injected with nutrient a second time. Each sample promptly reabsorbed approximately 20% of the gas evolved to that point. A duplicate of the active sample was heated at 160o C for 3 hr and, upon testing, gave a nil response. In additional controls, 60% less gas was evolved after heating a duplicate sample to 46.0°C. After another duplicate sample was heated to 51°C, the response became erratic and produced 90% less gas than did the active sample. Active Mars soils, enclosed in the Viking sample distribution box and held at approximately 10°C for 2 months at one site and 3 months at the other, showed no activity upon testing. In all, 9 tests were run. All of the Viking LR results6 at both sites are summarized in Table 1. Taken alone, the LR results were a strong positive indication of living microorganisms in the soil of Mars. The lability of the active agent in the soil to mild temperatures is difficult to explain otherwise. The difference between the responses after the heatings at 46.0°C and 51.5°C are reminiscent of laboratory microbiology analyses, such as the standard method7 for distinguishing fecal coliforms from the coliform group, which relies on small temperature differences. 2.3 Defects in non-biological interpretations of LR results Updating the last review8 with more recent information, each of the above enumerated arguments against a biological interpretation of the LR Mars data will be addressed. 1. No Organics: The Viking GCMS, designed to identify organic matter in the martian soil, failed to detect any at either Viking Lander site9. The presence of organic matter on Mars had been generally anticipated as a result of: photochemical synthesis from primitive atmospheric constituents10, synthesis from current martian atmospheric gases11,12 by UV, infall from comets and meteorites13 and from interplanetary dust particles14 (estimated15 at some 105 kg of organic carbon per year). The report of organic matter, including amino acids, inside the two SNC meteorites presumed from Mars, if verified, would confirm this anticipation. A report16 on EETA79001 found L-amino acid concentrations exceeding those for D-amino acids. Such chiral preference is found only in biological material. The finding17 of low molecular weight organic compounds being solar-stripped from the Hale-Bopp comet supports the widespread formation of organic matter. Nonetheless, the Viking GCMS negative results were widely accepted as persuasive "evidence" that the Viking samples contained some exotic chemistry, but no life18,19. However, the LR's finding20 of living microorganisms, as incorporated into FIG. 6, in Antarctic soil #726, in which the GCMS concurrently had detected no organic matter, provided a direct comparison of the two instruments. Moreover, a wet-chemistry analysis of Antarctic #726, performed as a control, found21 organic matter. Recently, the same soil, maintained in bonded storage by NASA, was re-examined22. The sample was subjected to prolonged digestion in concentrated HF/HCl and then pyrolyzed at 500°C (the highest pyrolysis temperature applied by the Viking GCMS). While the actual data were not presented, it was stated that the spectrum showed "weak bands in the 2850-3000 cm-1 characteristic of the C-H stretch of methylene groups (-CH2-). Consistent with this, (the) C2 (fraction) gave hydrocarbon fragments on pyrolysis and showed aliphatic (sp3) carbon in its 13C CP-MAS NMR spectrum." Thus, organic compounds surviving this rugged digestion were detected in the soil. They were attributed to kerogen and coal refractory to the GCMS pyrolysis. Biological possibilities were discounted although viable microorganisms have been reported23 within anthracite coal taken from deep underground. The purpose of the intensive digestion of the Antarctic #726 was not stated. One must wonder what variety of labile organic compounds were present prior to the digestion. When asked how the report of organic matter in ALH84001 could be reconciled with the negative results of the Viking GCMS, a NASA spokesman24 said that the GCMS had been sent to Mars long ago and may not have been sensitive enough to detect the amounts of organic matter in the low microorganism population in the meteorite. The Viking GCMS was reported25 to require organic content equivalent to 106 bacterial cells to obtain a response in its 100 mg soil sample. This is in sharp contrast to the 50-cell sensitivity of the LR. The difference in sensitivities can readily explain the disparate results reported by the two instruments. A fresh examination of the Viking Pyrolytic Release Experiment (PR) data26 supplies direct support for the formation and persistence of organic matter on Mars. In the experiment, a sample of soil is taken into a chamber containing simulated martian atmosphere with its CO and CO2 labeled with 14C. The sample is then exposed to simulated martian sunlight to permit any phototrophic microorganisms to photosynthesize and incorporate the label. After an incubation period, the non-incorporated gases are exhausted from the chamber, and the sample is pyrolyzed to release any labeled gas that had been fixed by the microorganisms. The detection of the gas released by the pyrolysis step is evidence for life. During the development of the PR instrument, the formation and accumulation of organic matter from CO and CO2 split by the UV of the simulated sunlight posed the serious threat of obtaining a false positive on Mars. The experimenters reported, "About 14 nmoles of 14C organics per gram of volcanic ash shale are formed within 70 hours of irradiation under a realistic simulation of martian conditions."27 This formation of organic matter from simulated martian atmosphere under simulated sunlight was independently confirmed28. The "solution" to the PR false positive problem was to incorporate an optical filter to screen out the UV below 320 nm. In its first experiment on Mars, the PR produced a net response of 81 cpm upon pyrolysis of the sample29. Extensive counting rendered a statistical significance exceeding 3 sigma above background and noise for this response. The response, nonetheless, was far short of the pre-mission criteria for life. The PR Experimenter concluded that the signal was of chemical origin. Seeking phototrophs, the PR was the most Mars-like experiment (no water was added). This made it difficult to get a response from terrestrial soils. Cultured microorganisms were frequently added to test soils to get a response. The PR operated on the presumption that martian organisms, under their native environment, would produce a response. Overlooked until now is the obvious fact that the low, but significant, PR response was from pyrolysis of the organic matter that had been created within the instrument on Mars. The UV filter did not completely eliminate the formation of organic matter. Pre-mission tests30,31 of the PR had shown that, even with the optical filter, organic matter accumulated on sterile test soils. The PR results on Mars indicate that the formation of organic matter is proceeding. 2. H2O2: The atmosphere of Mars produces H2O232,33. It was theorized34,35 that the H2O2 precipitates onto the Mars soil. The H2O2 and other oxidants produced from it were assumed to be in the soil sample and to have oxidized the LR organic substrates to release radioactive gas. It was proposed36 that the oxidant(s) also released the oxygen detected in the Viking GEx experiment. This oxidative chemistry also was credited with having cleansed the surface of Mars of any organic material, including microorganisms. The most telling evidence against the H2O2 theory is in a just-published report37 of very sensitive Earthbased IR telescopic measurements made through the entire column of the martian atmosphere. No spectrographic feature for H2O2 was found. The sensitivity of the instrument reduces the upper limit for H2O2 in the atmosphere of Mars to 30 ppb. Table 2 presents the amounts of martian H2O2 predicted by current leading H2O2 theorists. Sensitive to, or near, these projected amounts, the new experiment seriously weakens the H2O2 theories. Other unresolved difficulties have been reported38 for all theories attributing the LR Mars results to H2O2 under martian conditions. This chemical will oxidize the LR compounds to produce CO2. However relatively strong concentrations (approximately 2%) of H2O2 are required to emulate the amplitude and kinetics of the LR Mars positive responses. The most significant fact countering the H2O2 theory is that H2O2 and any of its proposed derivatives do not approximate the thermal sensitivity of the martian agent causing the LR responses. At 50°C, 90% H2O2 decomposes at only 0.001% per hour39. Of numerous attempts to simulate those results with H2O2, none reported has succeeded under conditions consistent with those on Mars. H2O2, highly sensitive to destruction by solar UV, has an expected lifetime in the martian atmosphere of less than one day. Any deposition of H2O2 from the atmosphere to the soil is likely too small to account for the LR results40. Based on the UV lability of H2O2, it is difficult for it to account for the LR activity. In addition, experiments41,42 reporting the formation of organic matter from simulated sun-irradiated martian atmosphere demonstrated that the organic matter accumulated despite the continuous incidence of the UV light. Evidently, the H2O2 formed under these simulated martian conditions was not made in sufficient quantity or did not survive the UV to oxidize the organic matter as fast as it was synthesized. The lifetime of H2O2 would be further reduced by the catalytic effects of y-Fe2O3 reported43 as likely to be extensive on Mars. The re-examination of the Mars PR data also has repercussions for the H2O2 theories. The theories propose that there is oxidant in the soil. Why did that oxidant not destroy the organics formed and detected within the PR? A variant chemical model proposing UV formation of peroxonitrites44 in the soil has been proposed since the review cited above. Aside from its failure to explain the result in the sample taken under the rock, this theory has not demonstrated the thermal sensitivity of the agent detected by the LR. Also, the formation of peroxonitrites would require amounts of nitrogen in the Mars soil in excess of the limit set by the Viking elemental analysis45. A general question about attributing the failure to find organics on Mars to peroxide is the finding46 of H2O2 in ancient permafrost cores on Earth, where the oxidant did not eliminate organic matter or life. Being nearer to the sun and having more water vapor than Mars does in its upper atmosphere, Earth experiences the formation of H2O2 in amounts greater than does Mars. Yet, H2O2 was not inimicable to the development of life and the accretion of organic matter on Earth. Life developed enzymes to protect against this oxidant, and many species came to utilize it in their energy-producing metabolic processes. 3. No Liquid Water; Extreme Environment: Next to the peroxide theories, the most vigorous defense of the chemical nature of the LR results stems from the beliefs that all life must have access to water in liquid form and that none is available on the surface of Mars. Moreover, it has been stated47 that the atmospheric pressure at the surface of Mars is below the triple point of water and likely has been so for several billion years. This is not supported by data obtained by the Infrared Interferometer Spectrometer (IRIS) experiment48 aboard the Mariner 9 Mars orbiter. It reported that "Extensive regions have been found where the surface pressure exceeds the triple-point pressure of water" and that "under temperature conditions which frequently occur on Mars, liquid water can exist in equilibrium with its vapor." A recent publication49 of Viking data provides a graph, FIG. 2, showing that, over a full martian year, the surface atmospheric pressures at both Viking landing sites continuously exceeded the triple point pressure for water. Further, inspection of the Viking data shows50 that the temperature of the top several millimeters of soil beneath the Viking Lander 2 sampling head rose to 273°K (the temperature at which ice liquifies) where it remained for at least several minutes. This is evidence for the melting of ice into liquid water. The thermal absorption of the metallic sampling head may have been responsible, but its thermal adsorption is likely equaled, or exceeded, by that of martian rocks. Surface temperatures in the southern latitudes at the start of summer were seen to exceed 273°K for several hours per day. Thermal inertias for large areas of Mars range above 6, and above 30 for bare rocks. Albedos of unfrosted martian ground are as low as 0.095.51 Viking orbital data52 also support the presence of liquid water in the upper few millimeters of the martian surface. FIG. 353 shows that, during the Viking primary mission period, large areas of the martian surface were above 273°K when an albedo of 0.25 and a thermal inertia of 6.5 were applied. Although the maximum temperature contour drawn for the Viking landing sites is 270°K, that surface temperature would be exceeded where, because of rocks or dark colorations, the albedo was less than 0.25 and/or the thermal inertia was greater than 6.5. Under these conditions, water would be in liquid form. "Thermal observations made half a century ago54,55,56 established that midday equatorial temperatures rose above the melting point of water."57 Within large geographic areas, the upper layer of the martian soil exceeds the triple point temperature and pressure for water which, thus, would occur in liquid form at least transitorily in diurnal and seasonal cycles. LR tests performed on the Death Valley sand dunes, shown in FIG. 4, detected microorganisms in the top one or two millimeters of the sand within one hour, as did tests performed on bare rock. Antimetabolites applied to duplicate samples as controls were effective in preventing evolution of gas, confirming the biological nature of the response. Samples of the top 2 mm of the sand were taken and analyzed by a NASA Jet Propulsion Laboratory (JPL) soil microbiologist. The moisture content and microbial populations reported58 were 0.9%, and 5.10 x 103 aerobic cells per gram, respectively. The above considerations address the presence of liquid water in terms of the more familiar terrestrial biological needs. Other factors concerning water and biology may apply. Even before the announcements of probable evidence for life in the martian meteorites, the possibility that life once existed on Mars was gaining favor.59 It was generally believed, however, that any such early life forms would have become extinct when liquid water was thought to have become unavailable. Alternatively, it was proposed, some organisms might have retreated to discrete "oases" deep below the surface where liquid water might still exist. Recent findings question the presumption that water activity on Mars is too low to permit aqueous moiety reactions. For example, chlorofluorocarbons are adsorbed onto stratospheric ice crystals which provide a liquid-like moiety for the chlorine to react with ozone.60 Such ice activation may accompany the diurnal frost depositions Viking observed on Mars, making biological reactions possible. Alternatively, water vapor rising from the Mars permafrost may be adsorbed on soil particles to form "double donor waters." These are reported to exist commonly in ice where they expose very large surfaces as thin films and clusters.61,62 An atomic force microscopy study63 of thin films of water indicates a solvating monolayer of phase I ice on adsorbing surfaces. This water might be available for biological reactions. Duracrust formations on Mars are attributed64 to the solvation and transportation of sulfate to the surface by the vapor flux from the Mars permafrost. This water of solvation might also accommodate biological reactions. Current availability of this solvating factor on Mars was indicated by the Viking GCMS results, which were cited65 as evidence for the availability of water to form the duracrust. Since Viking, life has been found thriving in extreme environments heretofore believed deadly, including high temperatures and pressures, and even in non-aqueous environments.66 A variety of microorganisms, including synergestic combinations such as lichen, live inside rocks in the dry valleys of the Antarctic67, where precipitation is rare and occurs only as light snow. Aseptically removed from within the rock, scrapings produced the positive LR response included in FIG. 6. Under extraordinarily harsh environments, many species of bacteria can enter a state in which they catabolize slowly, but neither grow nor divide68 until nutrient concentrations, temperature, and other conditions become favorable. A review69 cites many mechanisms for survival, including some for completely anhydrobiotic cells. Examples70,71 of dormant microorganisms indicate survivals approaching geologically significant time periods. Indeed, bacteria have been reported to have been resuscitated72 after 30 million years of dormancy under highly desiccating conditions. One of the surest methods of preserving bacteria indefinitely is to freeze-dry (lyophilize) them under conditions similar to those on Mars. Recovery from the resting cell state can be extremely rapid. An Oscillatoria strain subjected to drying in a desiccator above silica gel showed instantaneous recovery of photosynthesis upon resuscitation, with 100 percent viability.73 During the development of the Mars Oxidant Experiment (MOx)74, a test75 was conducted in which a California soil was dried to constant weight at 70°C. The end of an optical fiber was dipped into a glucose solution and dried, then was put into the dried soil and monitored for changes in reflectivity of the coated end to laser pulses sent through it as evidence for reaction. FIG. 5 shows the very rapid response showing that the glucose was being removed. A separate portion of the same soil pre-heated to 130°C produced no change in reflectivity confirming that the glucose had been removed by microorganisms. (A duplicate glucose-coated fiber put into solid potassium superoxide produced no reaction.) These results are reminiscent of the Viking LR data. The possibility exists that martian organisms may metabolize under present conditions or, long dormant, may have been resuscitated by the LR experiment. 4. "Too Much Too Soon": When the first positive result from the LR was obtained, it was stated that the response was "too much too soon" for microorganisms living under the extreme conditions of Mars. The Mars response was erroneously portrayed as exceeding the LR responses obtained from terrestrial soils and, therefore, indicative of chemistry. Were microorganisms present on Mars, the reasoning ran, they would be in far lesser numbers than in terrestrial soils. Hence, their response would be less, especially considering the harsh Mars environment. Furthermore, it was said that the response kinetics were indicative of chemistry, not biology. FIG. 6 compiles data directly comparing LR responses from a variety of terrestrial soils to the VL1 positive LR Mars response. The LR Mars response is seen to be near the lower end of the range, close to the Antarctic responses and to that from the Aiken soil in an LR flight instrument sealed within a chamber under the martian experiment conditions. The kinetics are seen to be similar. Thus, the "too much too soon" reason against acceptance of the LR response as biological is not supported by the relevant data. 5. 2nd Injection: LR tests with terrestrial soils generally show that, after a plateau in gas evolution has been reached, a second injection of the labeled nutrient produces a sharp renewal in gas evolution. While this was not one of the criteria for a determination of life on Mars, it would have lent support to such a determination. As seen in FIG. 7, when a second injection of nutrient was applied following the standard eight-day test cycle on Mars, approximately 20% of the gas previously evolved disappeared from the headspace of the instrument. This happened at both Viking sites. The gas slowly re-evolved to its eight-day level after approximately two months. A recent publication76 cites the lack of a vigorous evolution of gas following the second injection as the reason the LR results could not be from microorganisms. The second injection results seem to be the simple reabsorption of gas by the soil when wetted. In a laboratory simulation77, a Viking-type LR instrument containing sterilized Mars analog soil reproduced the reabsorption of approximately 20% of the headspace gas, FIG. 7 (shown with FIG. 1 for comparison). Furthermore, a search of the Viking development files revealed active soils which demonstrated the same lack of response, or slight reabsorption of gas, upon a second injection. Apparently, the microorganisms died during the test and the addition of water promoted reabsorption of the CO2 evolved after the first injection. Of particular interest are the results obtained when lichen were tested by the LR. The lichen responded very vigorously upon initial contact with the aqueous nutrient, but the rate of evolution quickly decreased and essentially ceased after only 24 hours. A comparison of the lichen incremental responses with those of the Mars LR is presented in FIG. 8. This suggests the possibility that too much water could have been a factor in both experiments. The above cited publication argues that increased gas evolution upon the second injection would have supported the biological nature of the response. However, had a second evolution of gas occurred, might not the argument have been made that water had become the limiting factor following the first injection, and that its resupply allowed a chemical reaction to continue? 6. No Visual Evidence: It was reported78 that a study made by the Viking Lander cameras showed no evidence for macro or micro forms of life. However, a detailed analysis of all the Viking Lander images79 revealed colored spots on many of the rocks and colored areas of the surface ranging from ochre to yellow to olive and green. A digital spectral analysis of the spots on the rocks as seen in six imaging channels (RBG and three near-IR) comported closely to that of images of lichen-bearing rocks. These rocks were viewed by the Viking cameras in the simulated Viking 1 Lander site and processed through the Viking Imaging System. The greenest objects in both fields of view were spots on the martian and Earth rocks. The respective digital numbers for hue, saturation and color for the spots in both images were similar. Subsequently, the greenish colors were independently confirmed80,81 with the additional observation that the green spots on the rocks were elevated by 0.1 to 1 mm above the background surface of the rock. While unusual weathering might account for the elevation of the spots and the changes observed, it has been suggested82 that lichen, or similar colonial microorganisms, could more readily satisfy the observations. Called "the pioneers of vegetation", lichen are the first life forms to appear on bare rock. Lichen have also been reported83 to subsist when the only source of water is atmospheric vapor. The atmosphere at both Viking Lander sites was at or near water vapor saturation diurnally, and frost was seen in many of the morning images. FIG. 9 is an image of three near-field rocks at Viking site 1 which were selected for study. The image was calibrated by the Viking Imaging System to provide the truest Mars colors. FIG. 10 is a heretofore unpublished set of 3 images encompassing most of the same area and taken at intervals of one martian year (two Earth years). All three images are treated identically, and closely approximate the calibration of FIG 9. The pictures are taken within several degrees of the same sunangle in order to permit comparison without mistaking light or shadow effects for true differences between successive images. The field of view was limited because of power conservation during the later portion of the extended mission. It is apparent that changes have occurred over time. Some of the near-field differences between the first two images were caused by sampling of the soil that occurred after the first image was taken. However, changes in both the near and distant fields cannot be associated with the sampling activity. A comparison of the same near-field fine surface structures among the three images shows that aeolian effects were essentially nil (the only apparent changes being from slumping that occurred around the dug hole). In particular, attention is directed at the foreground rock, which exhibits a major change in its patterned colored areas, and to the distant background where extensive pattern and color changes are evident. In several ways, then, the Viking Lander images provide evidence consistent with a biological interpretation of the LR data. 7. UV Light: This early hypothesis sought to explain both the LR active response and the lack of organics reported by the GCMS. An experiment at Viking Landing Site 2 eliminated this possibility. Before dawn, the sampling arm moved a rock before the sun's rays could strike it. A sample of soil that had underlain the rock and thus was protected from UV for eons was taken and promptly delivered to the instrument. Despite the absence of any UV exposure, the sample produced a positive LR response, essentially identical in kinetics and in magnitude to those of the Viking Lander 1 positive response seen in FIG. 1. This direct experiment carried out on Mars eliminated the possibility that UV light was the cause of the activity detected by the LR experiment on Mars. 8. Clays: It was proposed84,85,86 that clays on Mars reacted with the LR nutrient to produce labeled CO2 . Various clays were proposed in sequence as experiments detected deficiencies in duplicating the LR results. The clays, in turn, ranged from montmorillonite to nontronite to palagonite to smectites. The experiments suffered from basic problems87 including the failure to sterilize the samples tested or to use sterile procedures in the experiment in order to exclude biological effects from microorganisms in the clays. Extreme pHs were imposed to obtain responses and the responses did not have the kinetics of the LR positive responses. Data replicating the thermal sensitivities of the LR active agent at the lower levels of 50°C and 10°C were not forthcoming. Perhaps most important to the interpretations was the fact that the responses were not normalized to those from the Viking LR instrument. The counting efficiency used on the clays was 75% compared to 3% for the LR. Hence all responses for the clays should have been reduced by a factor of 25, which would have placed them in the control or noise levels. In the last work cited above, the claims of replicating the LR data were reduced to state that "iron-enriched smectites . . . were shown . . . to simulate many of the findings of the Viking Labeled Release Experiments on Mars." Considering that there is no direct evidence for clays on Mars, and the flawed nature of the experiments, the possibility that clays explain the LR Mars data seems vanishingly small. 9. Complexities of Independent Origin of Life: While listed last, this is really the penultimate reason responsible for discounting the LR Mars data as evidence for life. The origin of life is so poorly understood that the complexity posed by it alone would make the application of Ockham's Razor favor a non-biological explanation of the LR data. However, the presumed evidence of microbial life in the two martian meteorites raises the possibility that an independent origin of life on Mars was not necessary for life to exist on that planet. If the biological fossils are confirmed, the principle of panspermia will be established. EETA79001 is only 600,000 years old, in effect, of the "modern" era on Mars. The environmental changes deemed by many as inhospitable to life already had occurred. Were microorganisms alive so recently, their survival to the present would be readily inferred. The presence of microorganism fossils in the martian
meteorites would also make a case against the oases theory88
which proposes that microorganisms may currently exist only in pools of
liquid water postulated deep beneath the surface. Analysis89
of the physics of ejection of planetary material by meteoric impact concludes
that materials escaping planetary gravity originate from, or near, the
surface of the impact area. Thus, any fossils with the meteorites that
fell on Earth could not be from deep oases. Furthermore, were there deep
sites of living microorganisms, it is likely that volcanic action, frost
heaving, or other mechanisms would eject such material to the surface.
There any microorganisms could be widely distributed by the winds. The
microorganisms might adapt to or become lyophilized by the surface conditions.
Either way, they might still respond in the manner detected in the LR instrument
upon being moistened with liquid nutrient under benign conditions. Regardless,
the meteorites provide a now plausible vehicle for ejection into space,
lyophilization by the space environment, survival of re-entry, and thus,
interplanetary transportation of living microorganisms. The independent
origin of life on Mars is no longer a barrier to acceptance of the LR data
as evidence for life.
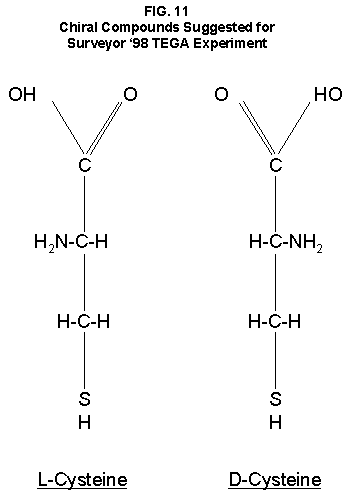
Many hypotheses have been advanced and tested in attempts to account for the well-characterized activity detected in the surface material of Mars by the LR experiment. As shown above, these hypotheses have themselves been found wanting. The demonstrated success of the LR and the exquisite sensitivity with which it has detected microorganisms during its extensive test program with its record of no false positives can no longer be denied. No non-biological approach published, or known to the author, has duplicated the LR Mars data. Some laboratory experiments have produced positive responses, but the detailed thermal sensitivity exhibited by the variety of controls conducted on Mars has remained elusive in all such tests compatible with martian conditions. On the other hand, a combination of known properties of microorganisms, perhaps even those possessed by single species, could reproduce all aspects of the LR data. The biological interpretation of the Mars LR results is left standing alone. Recent discoveries of life forms thriving in extraordinarily severe environments on Earth strongly indicate that any alien organisms arriving on Mars might well and widely adapt to their new home. Application of the scientific principle leads to a conclusion: the Viking LR experiment detected living microorganisms in the soil of Mars. 1. Add Life Detection Tests to Planned Missions. The above conclusion will require independent experimental confirmation before achieving general acceptance. No life detection experiments are planned for the NASA's 10 Mars landers scheduled over the next decade. However, it is still possible to add life detection capability to them within the new NASA paradigm of "cheaper, smaller, faster." Even without a dedicated life detection experiment, it is possible that confirmation of extant life may be achieved. Pathfinder is now enroute to Mars for a July 4, 1997 landing. Its lander is equipped with a camera having better color and spatial resolutions than did Viking's. It is possible that a close-up picture of a rock might show clear evidence of biological colonies. A search for such evidence should be a Pathfinder priority. 2. Use Hubble Telescope. The Hubble Telescope should be used to seek evidence of large areal changes in the color and patterns of the martian surface and seek to correlate them with atmospheric water vapor and climatic seasons. Useful images that already may exist in the Hubble files should be compared. 3. Seek Chirality in Mars Samples. Perhaps the surest robotic means for unequivocal distinction of biological from chemical reactions is a test for chiral activity. For some yet unknown reason, or by chance, when the first living cells came into existence their enzymes were chiral specific. They catalyzed protein-building reactions with L-amino acids only. They had a similar preference for L-carbohydrates over D-forms. Throughout the evolution of all living forms, these preferences have been genetically transmitted. This peculiarity of living systems provides a ready means of distinguishing them from chemical reactions. Chemical reactions, without the intervention of man, cannot distinguish between L- and D-isomers. On the other hand, all known life forms utilize and make virtually only the L-form of amino acids. Thus, were a sample of martian soil to react with one chiral isomer of a compound and not the other, the biological nature of the reaction would be established. A simple, small, low-cost experiment has been proposed90 which would separately add L-cysteine and D-cysteine (FIG. 11) to respective small ovens in the Surveyor '98 Thermal and Evolved Gas Analyzer (TEGA). TEGA will measure CO2 evolved when samples of martian soil placed in them are heated. Were the amount of CO2 evolved from the oven containing one isomer of cysteine to exceed the amount from the oven containing the other isomer significantly, the case for life would be confirmed. 4. Protect Health and Environment. Samples of martian material should not be returned to Earth before a thorough evaluation of possible hazards. The simple test for chirality could provide a warning of potential hazards to health and environment before a sample from Mars is returned to Earth. An upgraded version of the LR experiment might be added to an upcoming martian lander. This experiment would differ from the original in that the chiral isomers would be offered separately. The advantages of this approach are that it builds on the proven technology of Viking and that the Viking results provide relevant data for comparison. In addition, it could permit testing under controlled environmental conditions. Many other means of inquiry would follow a positive test to begin studies of comparatively biology. 5. Continue SNC studies. The ongoing appraisals
of the meteorites reported to contain evidence of martian microbial life
may confirm the conclusion reached herein. In particular, if EETA79001
is validated as to both its Mars origin and its evidence of microbial life,
this would constitute such confirmation.
In the 45 years since I first developed the radioisotopic method for detecting microorganisms, I have been fortunate in my assistants. This variously composed team total nearly half a hundred people. I have acknowledged each in the more than 50 published papers many have co-authored with me, and I now thank them collectively. I do want to mention several individuals. Dr. Patricia Ann Straat, my Viking Co-Experimenter, was my right arm at Biospherics during the 10-year period covering the Viking mission. She was essential to the success of the LR experiment. Her predecessor was Mary-Frances Thompson who did a splendid job overseeing our early laboratory efforts. Engineer George Perez was able to take my sketchy concepts for the early instruments and reduce them to excellently functioning metal. TRW, Inc. converted the early instruments into the faultlessly performing Viking LR instruments. I particularly want to thank my son, Dr. Ron Levin, a physicist, for his constant support of the LR life interpretation, his help with the water-on-Mars issue, and in extracting the Mars images from the JPL tapes. The bulk of the work reported on herein was funded
by a series of NASA Headquarters contracts with the companies where I was
employed: Resources Research, Inc., Hazleton Labs, Inc., and Biospherics
Incorporated. Since 1979, my efforts in analyzing the Viking Mission results
have been supported by Biospherics. I would like to thank its Board of
Directors for indulging my commitment to this issue and for the funds required.
1. McKay, D.S., E.K. Gibson, K.L. Thomas-Keptra, H. Vali, S. Romanek, S.J. Clemett, X.D.F. Chillier, C.R. Maechling, and N. Zare, "Search for Past Life on Mars: Possible Relic Biogenic Activity in Martian Meteorite ALH84001," Science, 273, 924-930, 1996. 2. "Martian Lanuch Pads," S&T's Weekly News Bull., Sky Pub. Corp., 1, 11, Nov. 96. 3. Levin, G.V.," Detection of Metabolically Produced Labeled Gas: the Viking Mars Lander," Icarus, 16, 153-166, 1972. 4. Levin, G.V. and P.A. Straat, "Labeled Release - An Experiment in Radiorespirometry", Origins of Life, 7, 293-311, 1976. 5. Miller, S.L., "A Production of Amino Acids Under Possible Primitive Earth Conditions," Science, 117, 528, 1953. 6. Levin, G.V. and P.A. Straat, "A Reappraisal of Life on Mars," Proc.. NASA Mars Conf., Nat. Ac. Sci., ed. D. Reiber, Am. Astronaut. Soc., San Diego, 1988. 7. Standard Methods for the Examination of Water and Wastewater, Am. Pub. Health Assn., Washington, D.C., 1992. 8. Op. Cit. 6. 9. Biemann, K., J. Oro, P. Toulmin III, L.E. Orgel, A.O. Nier, D.M. Anderson, P.G. Simmonds, D. Flory, A.V. Diaz, D.R. Rushneck, J.E. Biller and A.l. Lafleur, "The Search for Organic Substances and Inorganic Volatile Compounds in the Surface of Mars," J. Geophys. Res., 82, 4641, 1977. 10. Op. Cit. 5. 11. Hubbard, J.S., J.P. Hardy and N.H. Horowitz, "Photocatalytic Production of Organic Compounds from CO and H2O in a Simulated Martian Atmosphere," Proc. Nat. Acad. Sci., 3, 574, 1971. 12. Farris, J.P. and C.T. Chen, "Chemical Evolution. XXVI. Photochemistry of Methane, Nitrogen, and Water Mixtures as a Model for the Atmosphere of the Primitive Earth," J. Am. Chem. Soc., 97, 2962, 1975. 13. Biemann, K., "The Implications and Limitation of the Findings of the Viking Organic Analysis Experiment," J. Mol. Evol., 14, 68, 1979. 14. Anders, E., "Pre-biotic Organic Matter from Comets and Asteroids," Nature, 342, 255, 1989. 15. Sagan, C., personal communication. 16. Wright, I.P., M.M. Grady and C.T. Pillinger, "Evidence Relevant to the Life on Mars Debate, (2) Amino Acid Results," Lunar and Planet. Sci., XXVIII, 1587-1588, 1997. 17. Biver, N., Bockelee-Morvan, P. Colom, J. Crovisier, J.K. Davies, W.R.F. Dent, D. Depois, E. Gerard, E. Lellouch, H. Raver, R. Moreno and G. Paubert, "Evolution of the Outgassing of Comet Hale-Bopp (C/1995 01) from Radio Observations," Science, 275, 1915-1918, 1997. 18. Klein, H.P., "The Viking Biological Experiments on Mars," Icarus, 34, 666, 1978. 19. Zendt, A.P. and C.P. McKay, "The Chemical Reactivity of the Martian Soil and Implications for Future Missions," Icarus, 108, 146, 1994. 20. Op. Cit. 4. 21. Lavoi, J.M., Jr., Support Experiments to the Pyrolysis/Gas Chromatograph/Mass Spedtrometric Analysis of the Surface of Mars, Ph.D. Thesis, p. 252, MIT, Feb. 1979. 22. Chronin, J.R., "Organic Carbon of Antarctic Soil #726: A Viking Footnote," Dept. Chem. and Biochem., Arizona State U., Nov. 16, 1995. 23. Lipman, C. B., "Living Microorganisms in Ancient Rocks," J. Bact., Vol. XXII, No. 3, 183-198, 1931. 24. Huntress, Wessly, speaking at NASA press conf., NASA HQ, Washington, Aug., 1996. 25. Biemann, K., Viking Science Discussion, JPL, Aug. 1976. 26. Horowitz, N.H. and G.L. Hobby, "Viking on Mars: The Carbon Assimilation Experiments," J. Geophys. Res., 82, 4659, 1977. 27. Hubbard, J.S., J.P. Hardy, G.E. Voecks and E.E. Golub, "Photocatalytic Synthesis of Organic Compounds from CO and Water: Involvement of Surfaces in the Formation and Stabilization of Products", J. Mol. Evol., 2, 149-166, 1973. 28. Op. Cit. 12. 29. Klein, H.P., N.H. Horowitz, G.V. Levin, V.I. Oyama, J. Lederberg, A. Rich, J. Hubbard, G.L. Hobby, P.A. Straat, B.J. Berdahl, G.C. Carle, F.S. Brown, and R.D. Johnson, "The Viking Biological Investigation: Preliminary Results," Science, 194, 4260, 1976. 30. Op. Cit. 11. 31. Op. Cit. 27. 32. Hunten, D., "Possible Oxidant Sources in the Atmosphere and Surface of Mars," J. Mol. Evol., 14, 57, 1979. 33. Hunten, D., 4.7, "Oxidants in the Martian Atmosphere and Soil," JPL Pub. D-4657, 1987. 34. Oro, J. Presentation to Viking Science Team, JPL, Aug. 1, 1976. 35. Oyama, V.I. and B.J. Berdahl, "A Model of Martian Surface Chemistry," J. Mol. Evol., 14, 199, 1979. 36. Op. Cit. 34. 37. Krasnopolsky, V., G.L. Bjoraker, M. J. Mumma and D. E. Jennings, "High-resolution spectroscopy of Mars at 3.7 and 8.4 µm: A sensitive search for H2O2, H2CO, HCl, and CH4, and detection of HDO, J. Geophys. Res., 102, No. E3, 6525-6534, 1997. 38. Levin, G. V. and P. A. Straat, "A Search for a Nonbiological Explanation of the Viking Labeled Release Life Detection Experiment," Icarus, 45, 494-516, 1981. 39. Schumb, W.C., C.N. Satterfield and R.L. Wentworth, in Hydrogen Peroxide, p. 520, Am. Chem. Soc. Monograph Series, Reinhold Pub. Corp., NY, 1995. 40. Bullock, M.A., C.R. Stoker, C.P. McKay and A.P. Zent, "A Coupled Soil-Atmosphere Model of H2O2 on Mars," Icarus, 107, 142, 1994. 41. Op. Cit. 11. 42. Op. Cit. 12. 43. Hargraves, R.B., D.W. Collinson, R.E. Arvidson and C.R. Spitzer, "The Viking Magnetic Properties Experiment: Primary Mission Results," J. Geophys. Res., 82, 4547, 1977. 44. Plumb, R., R. Tantayanon, M. Libby and W.W. Xu, "Chemical Model for Viking Biology Experiments: Implication for the Composition of the Martin Regolith," Nature, 338, 633, 1989. 45. Clark, B.C., A.K. Baird, H.J. Rose, P. Toulmin III, R.P. Christian, W.C. Kelliher, A.J. Castro, C.D. Rowe, K. Kiel and G.R. Huss, "The Viking X Ray Fluorescence Experiment: Analytical Methods and Early Results," J. Geophys. Res., 82, 4577, 1977. 46. Neftel A., P. Jacob and D. Klockow, Tellus, 38B, 262, 1986. 47. McKay, C.P. and C.R. Stoker, "The Early Environment and Its Evolution on Mars," Rev. Geophys., 27, 189, 1989. 48. Hanel, R., B. Conrath, W. Hovis, V. Kunde, P. Lowman, W. Maguire, J. Pearl, J. Pirraglia, C. Prabhakara, B. Schlachman, G. Levin, P. Straat, and T. Burke, "Investigation of the martian Environment by Infrared Spectroscopy on Mariner 9," Icarus, 17, 423, 1972. 49. Carr, M. H., Water on Mars, Figure 1-2, P 10, Oxford University Press, New York, 1996. 50. Moore, H.J. et al, "Surface Materials of the Viking Landing Sites," J. Geophys. Res, 82, No. 28, 4497-4523, 1977. 51. Kieffer, H. H., T. Z. Martin, A. R., Peterfreund, B. M. Jakosky, E. D. Minor, and F. D. Palluconi, "Thermal and Albedo Mapping of Mars During the Viking Primary Mission," J. Geophys. Res., 82, 4249-4291, 1977. 52. Op. Cit. 51, Figure 18a. 53. Ibid. 54. Coblentz, W.W., "Further Measurement of Stellar Temperatures and Planetary Radiation," Proc. Nat. Acad. Sci., 8, 330, 1922. 55. Coblentz, W.W. and C.O. Lampland, Sci. Pap. Bur. Stand., 22, 237, 1927. 56. Pettit, E. and S.B. Nicholson, "Measurements of the Radiation From the Planet Mars," Pop. Astron., 32, 601, 1924. 57. Op. Cit. 51. 58. Levin, G.V. and A.H.Heim, in Life Sciences and Space Research III, M. Florkin, ed., North Holland Pub. Co., 1965. 59. McKay, C.P., R.L. Mancinelli, C.R. Stoker, and R.A. Wharton, Jr., in Mars, pp. 1234-1245, Keiffer, H.H., B.M. Jakosky, C.W. Snyder, M.S. Mathews, eds., U. Ariz. Press, Tucson, 1992. 60. Sander, S.P., R.R. Friedl and Y.L. Yung, "Rate of Formation of the CIO Dimmer in the Polar Stratosphere: Implications for Ozone Loss," Science, 245, 1095, 1989. 61. Pribble, R.N. and T.S. Zwier, "Size-Specific Infrared Spectra of Benzene-(H2O)n Clustersw (n = 1 through 7): Evidence for Noncyclic (H2O)n Structures," Science, 265, 75, 1994. 62. Li, J. and D.K. Ross, "Evidence for Two Kinds of Hydrogen Bond in Ice," Nature, 365, 327, 1993. 63. Hu, J., X.-D. Xiao, D.F. Ogletree and M. Salmeron, "Imaging the Condensation and Evaporation of Molecularly Thin Films of Water with Nanometer Resolution," Science, 268, 267, 1995. 64. Toulmin, P. III, A.K. Baird, B.C. Clark, K. Keil, H.J. Rose, R.P. Christian, H.P. Evans and W.C. Kelliher, "Geochemical and Mineralogical Interpretation of the Viking Inorganic Chemical Results," J. Geophys. Res., 82, 4625, 1977. 65. Ibid. 66. Flam, F., "The Chemistry of Life at the Margins," Science, 265, 471, 1994. 67. Friedmann, E.I. and R. Ocampo, "Endolithic Blue-Green Algae in the Dry Valleys: Primary Producers in the Antarctic Desert Ecosystem," Science, 193, 1247, 1976. 68. Kjelleberg, S., M. Hermansson and P. Mardin, "The Transient Phase Between Growth and Nongrowth of Heterotrophic Bacteria with Emphasis on the Marine Environment," Ann. Rev. Microbiol., 41, 25-47, 1987. 69. Potts, M., "Desiccation Tolerance of Prokaryotes," Microbiol. Revs., 58, 755, 1994. 70. Gest, H. and J. Mandelstam, "Longevity of Microorganisms in Natural Environments," Microbiol. Sciences., 4(3), 69, 1987. 71. Gilichinsky, D.A., E.A. Vorobyova, L.G. Erokhina, D.G. Fyordorov-Dayvdov and N.R. Chaikovskaya, "Life Science and Space Research XXIV(3): Planetary Biology and Origins of Life, Proceedings of the Topical Meeting of COSPAR Interdisciplinary Scientific Commission F (Meetings F7, F1, F8 and F9) and Evening Session 1 of the COSPAR Twenty-eighth Plenary Meeting," Adv. Space Res., 12(4), 255, 1992. 72. Cano, R.J. and M.K. Borucki, Science, 268, 1060, 1995. 73. Op. Cit. 69. 74. Grunthaner, F.J., A.J. Ricco, M.A. Butler, A.L. Lane, C.P. McKay, A.P. Zent, R.C. Quinn, B.R. Murray, H.P. Klein, G. V. Levin, R.W. Terhune, M.L. Homer, A. Ksendzov and P. Niedermann, Analyt. Chem., in press, 1995. 75. Suggested by G.V. Levin, conducted by A. Ksendzov, MOx Team members, as part of MOx development program, Apr., 1993. 76. Goldsmith, D., The Hunt for Life on Mars , p. 188, Dutton, NY, 1997. 77. Levin, G. V., and P. A. Straat, "Laboratory Simulations of the Viking Labeled Release Experiment: Kinetics Following Second Nutrient Injection and the Nature of the Gaseous End Product," J. Mol. Evol., 4, 185-197, 1979. 78. Levinthal, E.C., K.L. Jones, P. Fox and C. Sagan, "Lander Imaging As A Detector Of Life On Mars," J. Geophys. Res., 82, 4468-4478, 1977. 79. Levin, G.V., P.A. Straat and W.D. Benton, J. Theoret. Biol., 75, 381-390, 1978. 80. Guinness, E., R. Arvidson, L. Geheret and L. Bolef, "Color Changes at the Viking Landing Sites over the Course of a Mars Year," NASA Technical Memorandum 80339, 1979. 81. Strickland, E. L. III, "Martian soil stratigraphy and rock coatings observed in color-enhanced Viking Lander images," Proc. 10th Lunar Planet. Sci. Conf., 3055-3077, 1979. 82. Op. Cit. 6. 83. Lange, C., E. Schulze and W. Koch, Flora Abt., 159, 38, 1970. 84. Banin, A. and J. Rishpon, "Smectite clays in Mars soil: evidence for their presence and role in Viking biology experimental results," J. Molec. Evol., 14, 133-152, 1979. 85. Banin, A. and L. Margulies, "Simulation of Viking biology experiments suggests smectites not palagonite, as martian soil analogs," Nature, 305, 523-526, 1983 86. Banin, A., T. Ben-shlomo, L. Margulies and A.U. Gehring, "Workshop on the Martian Surface and Atmosphere Through Time," NTIS, Accession No. N92-28990/9/XAB, 1992. 87. Op. Cit., 38. 88. McKay, C.P., R. L. Mancinelli, C. R. Stoker, and R. A. Wharton, Jr., "The Possibility of Life on Mars During a Water-Rich Past," Mars, 1234-1245, Keiffer, H. H., B. M. Jakosky, C. W. Snyder, M. S. Mathews, eds., U. Ariz. Press, Tucson, 1992. 89. Gratz, A.J., W.J. Nellis and N.A. Hinsey, "Observations of High-Velocity, Weakly Shocked Ejecta from Experimental Impacts," Nature, 363, 522, 1993. 90. Levin, G.V., letter proposal to W. Boynton, TEGA Experimenter, U. Ariz., Jan. 19, 1996. |
FIGURES



TABLES


| Life on Mars Main | Order Papers | Mars Pictures | Viking Mission | Comments and Questions |